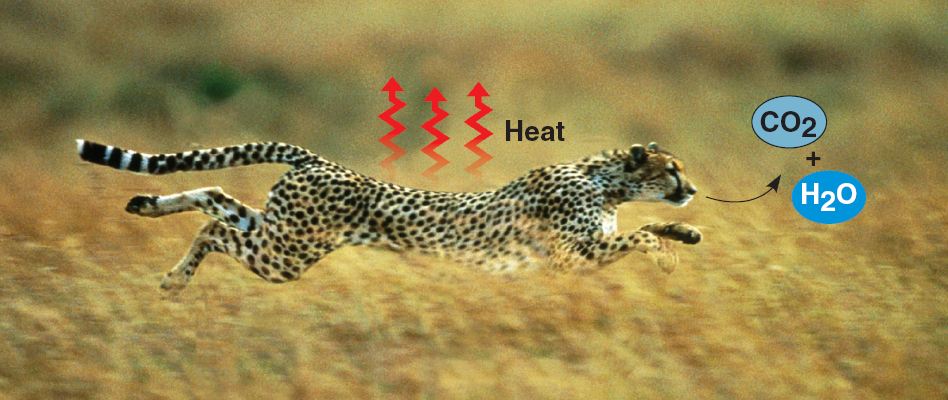
Second law of thermodynamics.
Every energy transformation increases the disorder (entropy) of a closed
system.
For example, entropy is added to the cheetah's surroundings in the form of heat
and the small molecules that are the by-products of metabolism.
