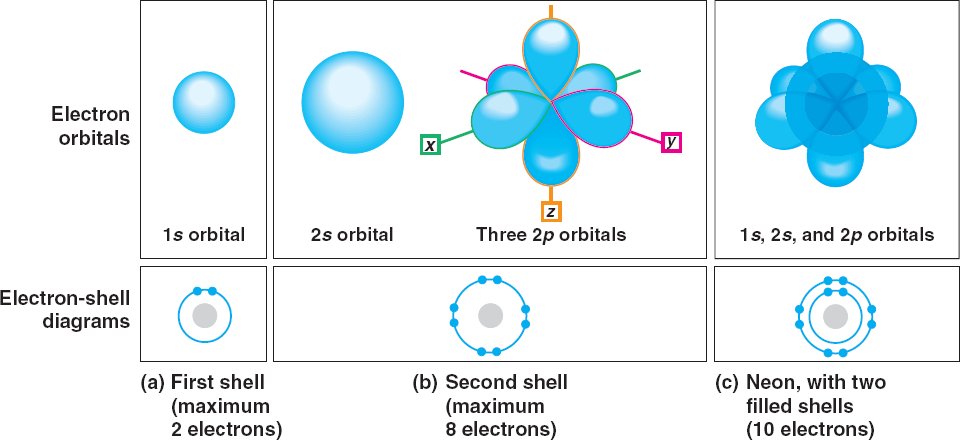
Electron orbitals.
Each orbital
(volume of space where the electrons of an atom are most likely to be found) holds a maximum of 2 electrons.
An electron shell represents the average distance of an electron from the nucleus.
- The first electron shell has one spherical (1s) orbital.
- The second shell has one larger s orbital (2s) plus three dumbbell–shaped orbitals (2p).
- Neon has 10 electrons.
